Abstract
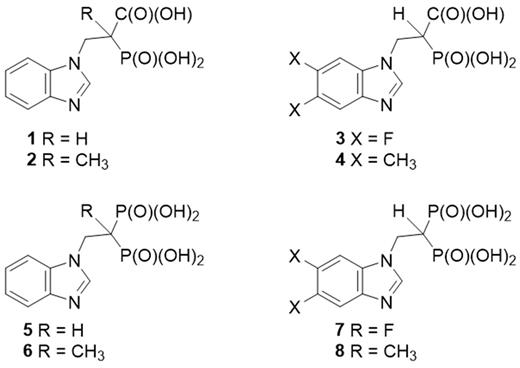
Malignant plasma cells are characterized by the production of monoclonal protein (MP). Our work has demonstrated that disrupting intracellular MP trafficking by targeting the geranylgeranylation of Rab GTPases results in endoplasmic reticulum stress, induction of the unfolded protein response pathway and apoptosis of myeloma cells. Rab proteins are geranylgeranylated by the enzyme geranylgeranyl transferase II (GGTase II) which uses geranylgeranyl diphosphate as the isoprenoid substrate. We have pursued the development of specific GGTase II and geranylgeranyl diphosphate synthase (GGDPS) inhibitors as potential anti-myeloma agents. A family of compounds incorporating a benzimidazole heterocycle and either a carboxyphosphonate or bisphosphonate was prepared by modification of the appropriate heterocycles. The core benzimidazole phosphonate structure was modified with substituents at either the alpha-carbon position or at the 5 and 6 positions on the benzimidazole (Figure). These compounds were subjected to in vitro enzyme assays for GGTase II and the related prenyltransferases farnesyl transferase (FTase) and GGTase I as well as the synthases farnesyl diphosphate synthase (FDPS) and GGDPS. Cellular disruption of protein prenylation was assessed via immunoblot analysis for Ras (a representative farnesylated protein) and for unmodified Rap1a (a representative geranylgeranylated protein) in human myeloma cells (RPMI-8226, MM.1S). The ability of the novel compounds to disrupt MP trafficking was determined by measuring changes in intracellular lambda light chain levels via ELISA. Confirmation of disruption of Rab geranylgeranylation was achieved by Triton X-114 lysis to obtain detergent (membrane) fractions which were subjected to Rab6 (a representative Rab protein) immunoblot analysis. These studies demonstrated that the nature of the phosphonate head group has a profound impact on enzyme specificity: the carboxyphosphonates have specific activity as GGTase II inhibitors while the bisphosphonate analogues have specific activity as FDPS inhibitors with no cross-reactivity against any of the other tested enzymes. The most potent GGTase II and FDPS inhibitors were the unsubstituted analogues. The carboxyphosphonate 1 was found to have an IC50 of 230 μM against GGTase II and cellular activity starting at 2.5 mM. This represents a two-fold improvement in potency compared with the known GGTase II inhibitor 3-PEHPC. The bisphosphonate 5 has an IC50 of 24 nM against FDPS and cellular activity at ≥ 50 μM. The addition of a methyl group at the alpha-carbon position abrogated the activity of the carboxyphosphonate (2) as a GGTase II inhibitor and the activity of the bisphosphonate (6) as a FDPS inhibitor. The addition of substituents at the 5 and 6 positions (compounds 3, 4, 7 and 8)did not improve potency compared with the parent compounds. Co-incubation with low concentrations of the HMG-CoA reductase inhibitor lovastatin (0.1-1 μM), concentrations which on their own are not sufficient to disrupt protein prenylation, potentiated the cellular activity of the lead GGTase II and FDPS inhibitors. In conclusion, these benzimidazole phosphonates represent novel classes of GGTase II and FDPS inhibitors. These studies highlight the importance of the identity of the phosphonate head-group in dictating enzyme specificity. Further studies are underway to prepare additional benzimidazole analogues in order to improve their potency, advance the understanding of the structure-function relationship, and assess the potential of these compounds as anti-myeloma agents.
Wiemer: Terpenoid Therapeutics, Inc: Other: Founder. Holstein: Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.